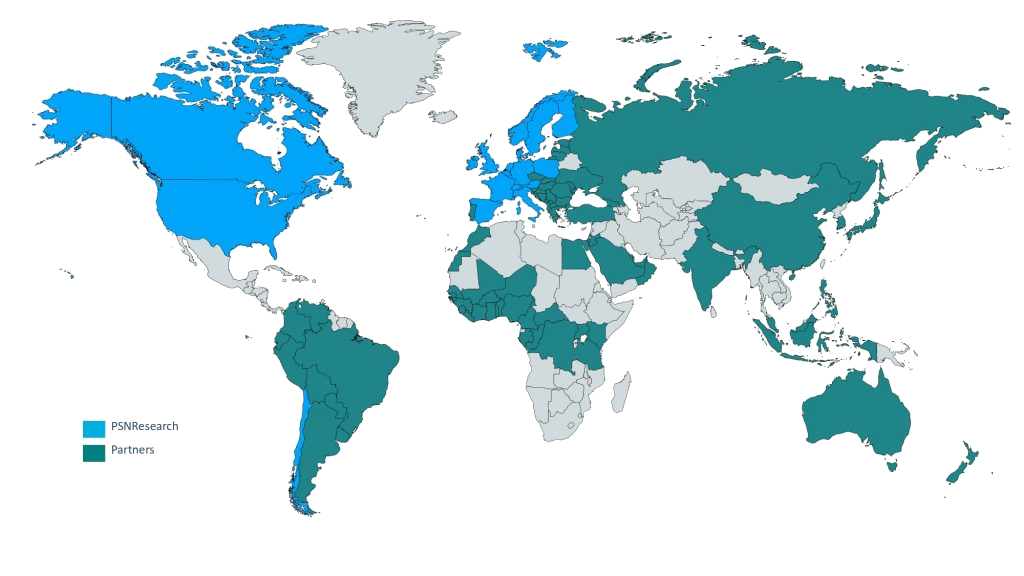
PSNResearch is a unique international clinical CRO model, offering full-service support to medical device manufacturers, biotechs, medtechs and pharmaceutical firms developing interventional and non-interventional, exploratory & confirmatory clinical trials, including also real-world post-authorization studies (Market Access / HEOR, secondary databases, etc.).
With 440 regional experts and 13 official offices in North & South America and Europe, including Lyon for France, our organization is a personalized alternative to large CROs and offers the highest professional added value. PSNResearch is large enough to cover any type of clinical development program, but the right size to give the attention and flexibility that each of your international projects deserves.