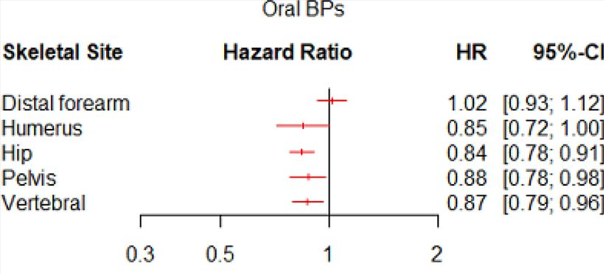
The SNDS-PMO study, conducted by RCTs using the French healthcare database system (Système National des Données de Santé (SNDS)), evaluate the risk of fragility fracture after long-term discontinuation of osteoporosis treatments in postmenopausal women. We identified 81,263, 19,111, and 28,606 women who initiated oral bisphosphonates, intravenous bisphosphonates, and denosumab, respectively, during the selection period: 55%, 69%, and 43% experienced long-term discontinuation. The results show that long-term discontinuation significantly increases the risk of fragility fractures compared to women who do not discontinue treatment (matched by repeated propensity scores over time), by 12% for oral bisphosphonates and by 92% for denosumab.
This study, presented orally at the EULAR 2024, DSVR 2024, and ADELF-PITER 2024 congresses, was the first in France to evaluate the practices of long-term discontinuation of osteoporosis treatments in almost the entire population of treated women in France. It demonstrates a concrete application of the dynamic pharmaco-epidemiology methods developed by RCTs using medico-administrative data to reconstruct treatment patterns.
N° T74497032022011 (https://www.health-data-hub.fr/projets/arret-du-traitement-anti-osteoporotique-chez-les-femmes-menopausees-atteintes-dosteoporose)
Data Controller: Amgen
Data Processor: RCTs
Scientific Committee: Karine Briot
Plus d’information : https://ard.bmj.com/content/83/Suppl_1/144