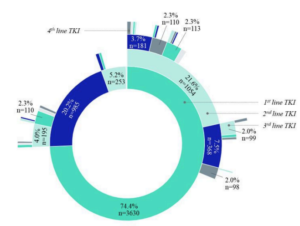
Over the past decade, the treatment of Chronic Myeloid Leukemia (CML) has been revolutionized by the introduction of tyrosine kinase inhibitors (TKI). For patients achieving a deep molecular response, stopping TKI treatment – treatment-free remission (TFR) – becomes a possibility. This study, conducted by RCTs, aimed to develop predictive models for the success or failure of treatment-free remission based on clinical data coupled with French healthcare database system (Système National des Données de Santé (SNDS)). This indirect linkage (i.e., using common variables between the two databases) was carried out by RCTs using a deterministic matching algorithm developed by the RCTs secondary data team. For prediction, logistic regression models and machine learning algorithms were tested. This study provides an example of developing predictive models/decision support tools, developed from patients’ clinical data enriched with SNDS data.
N° T40786692021052 (https://www.health-data-hub.fr/projets/real-life-treatment-patterns-and-burden-illness-among-patients-chronic-myeloid-leukemia-cml)
Data Controller: Novartis
Data Processor: RCTs
Scientific Committee: Franck-Emmanuel Nicolini
Risk Factors TFR CP-CML Risk Factors TFR CP-CML